• What is GasSimTM?
• How to use GasSim
• Ideal and Real Gas Models
• Examples
• References
GasSim is a gas law modeling and simulation tool. It is a web app that is freely available to all students and teachers. GasSim is intended for undergraduate and high-school students in chemistry or physics courses.
GasSim comes with a database of properties for 76 gases, including elements, inorganic compounds, hydrocarbons, and organic compounds. GasSim provides a simple interface that allows the user to vary the temperature, volume or the number of moles of a selected gas, and evaluates the pressure as a function of the chosen simulation variable. The three independent variables represent the three ways that a confined gas can be manipulated in an actual lab: by heating/cooling the gas; by compressing/uncompressing the gas (for example, by using a piston); by adding/removing gas molecules (for example, by using a syringe). The simulator then computes the pressure using three different gas models: the ideal gas model, the van der Waals real gas model, and the Peng-Robinson real gas model. Results are provided in both graphical and tabular forms.
GasSim is designed to serve as a virtual lab that students can use to experiment with and develop a deeper understanding of ideal and real gas laws. Students can also compare the ideal gas behavior to the two real gas models that are more realistic.
1. Select a gas using the searchable drop-down list on the top-left corner of the dashboard on the "Simulator" page.
2. Select a simulation variable using the second drop-down list -- this is the variable that will varied in the simulation. The available choices are the three independent variables: temperature, volume, and the number of moles of the gas.
3. Use the sliders to set minimum and maximum values for the simulation variable, and fixed values for the other two independent variables. For example, if volume is the simulation variable, then use the first two sliderst to set the minimum and maximum volumes, and the other sliders to set fixed values for the temperature and the number of moles. If the slider does not work on touch-enabled devices, enter the values directly into the text boxes.
4. Click "Simulate" to run the simulation. The results will be depicted in graphical and tabular forms, comparing the pressures calculated using all three gas models as a function of the simulation variable.
5. The data table can be copied and pasted into Excel for further graphing or analysis (including emprical curve fitting) or just to change the results to different units. Data from multiple simulations can be also be compared using Excel.
6. The x-axis range in the chart is based on the minimum/maximum values of the simulation variable. In order to zoom in or out on x-axis of the chart, adjust those minimum/maximum values using the sliders and re-run the simulation.
Ideal gas model:
P Vm = R T,
P = pressure (bars), Vm = molar volume (L/moles), T = temperature (K), and R is the gas constant.
van der Waals model for "real" gases:
( P + a / Vm2 )( Vm - b ) = R T
a = 27 R2 Tc2/(64 Pc)
b = R Tc/(8 Pc)
Tc = critical temperture, Pc = critical pressure.
Peng-Robinson model for "real" gases:
P = R T/(Vm - b ) - a α/(Vm2 + 2bVm - b2)
a = 0.45724 R2 Tc2/Pc
b = 0.0778 R Tc/(8 Pc)
α = (1 + k (1 - Tr0.5))2
k = 0.37464 + 1.54226 ω - 0.26992
ω2
Tr = T/Tc, ω = acentric factor.
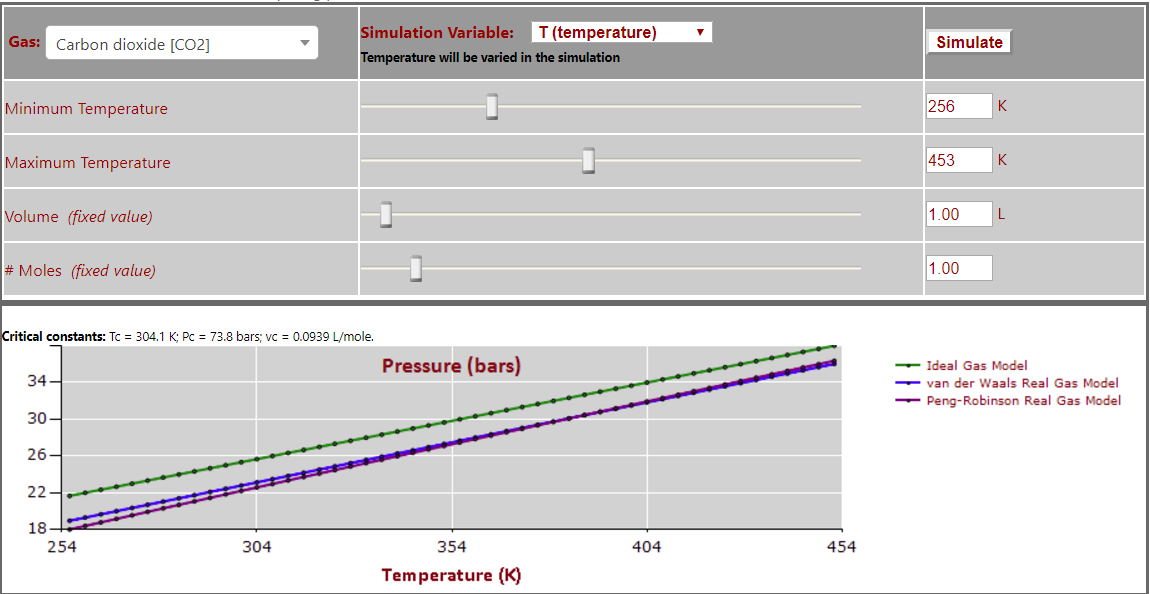
Pressure vs. Temperature (note nearly linear relationship) for CO2:
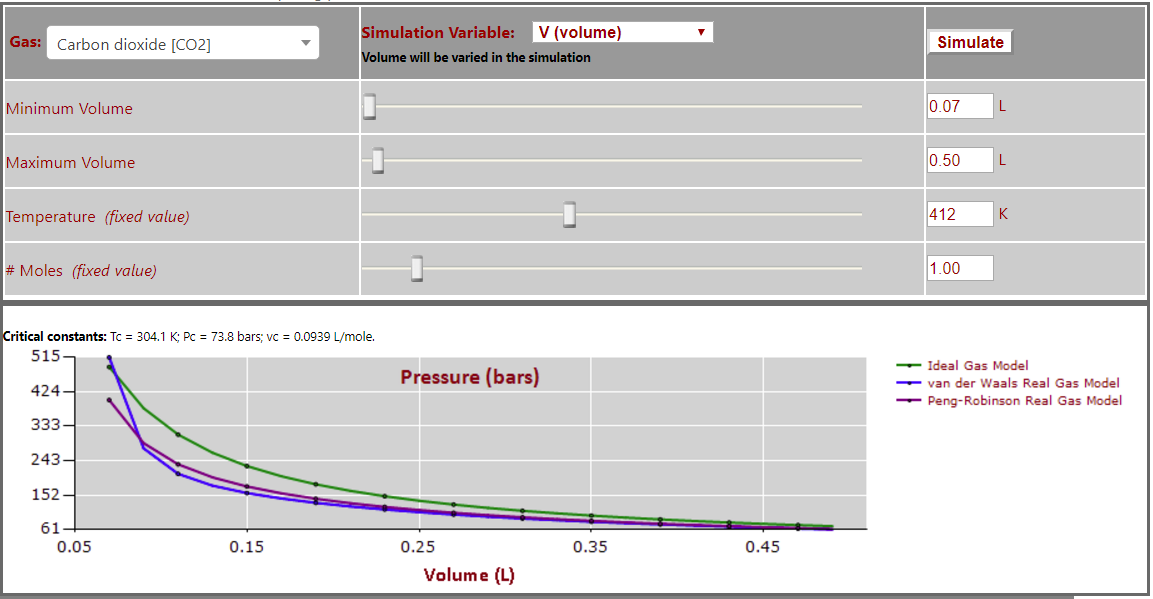
Pressure vs. Volume (T > Tc - note expected inverse P-V relationship) for CO2:
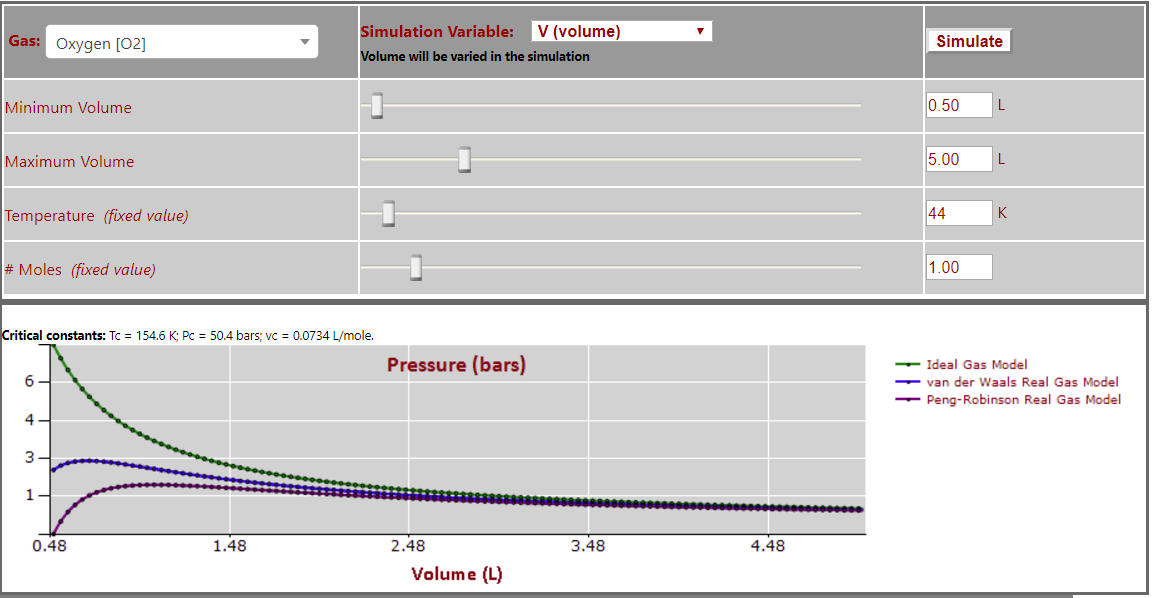
Pressure vs. Volume (T < Tc - note oscillations at low volumes) for O2:
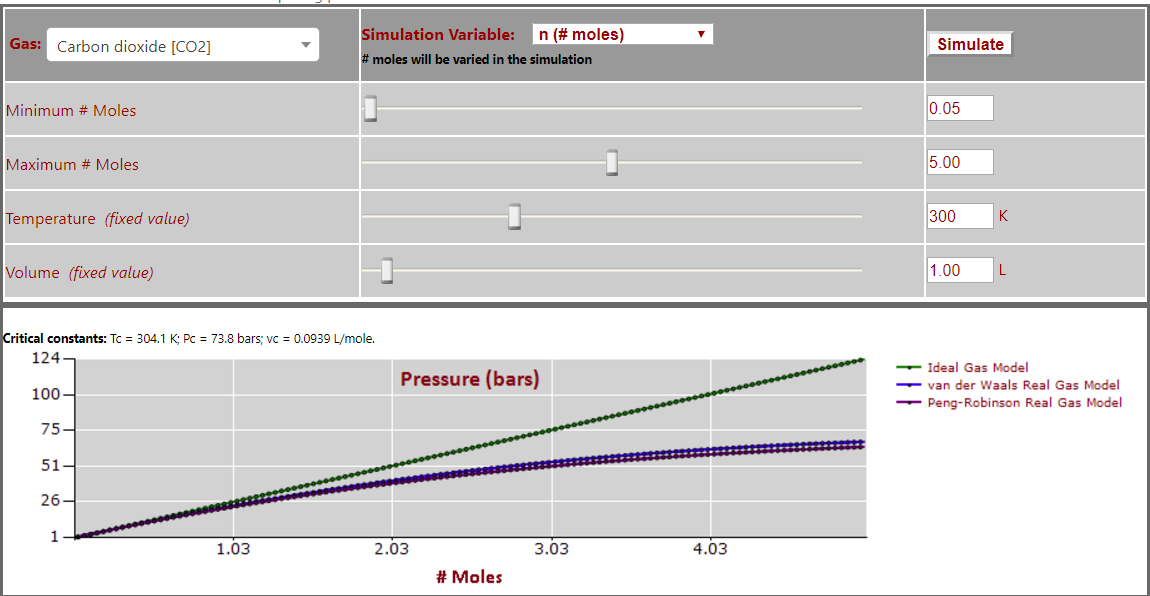
Pressure vs. Number of Moles (note leveling off of pressure at high gas density) for CO2:
-
Pauling, L. 1988. General Chemistry. New York: Dover Publications.
-
Atkins, P. and De Paula, J. 2014. Physical Chemistry: Thermodynamics, Structure, and Change. New York: W.H. Freeman & Co.
-
Prausnitz, J.M., Lichtenthaler, R.N., and de Azevedo, E. G. 1999. Molecular Thermodynamics of Fluid-Phase Equilibria. Upper Saddle River, NJ: Prentice-Hall.
-
Young, D. Equations of State. Cytoclonal Pharmaceutics Inc.